Chromium hydride
|
Read other articles:

For the neighbourhood in Düsseldorf, see Düsseldorf-Niederkassel. Town in North Rhine-Westphalia, GermanyNiederkassel Town Coat of armsLocation of Niederkassel within Rhein-Sieg-Kreis district Niederkassel Show map of GermanyNiederkassel Show map of North Rhine-WestphaliaCoordinates: 50°49′N 7°02′E / 50.817°N 7.033°E / 50.817; 7.033CountryGermanyStateNorth Rhine-WestphaliaAdmin. regionKöln DistrictRhein-Sieg-Kreis Subdivisions7Government • Mayor (20…
Arena of Valor Logo Arena of Valor versi rilis globalBerdasarkanHonor of Kings Publikasi26 November 2015[1]GenreArena pertarungan daring multipemainKarakteristik teknisSistem operasiAndroid dan iOS PlatformiOS, Android dan Nintendo Switch MesinUnity (mesin permainan) Modepermainan video multipemain Formatunduhan digital Metode inputlayar sentuh Format kode Daftar 30 Informasi pengembangPengembangTiMi Studio, GarenaPenyuntingTencent Garena PenerbitTencentPenilaianESRB USK Sumber kode Goog…

Halaman ini berisi artikel tentang ordo militer Katolik berdaulat. Untuk kegunaan lain, lihat Ordo Santo Yohanes (disambiguasi) dan Ksatria Malta (disambiguasi). Ordo Hospitaler Militer Berdaulat Santo Yohanes dari Yerusalem, Rodos, dan MaltaSovrano Militare Ordine Ospedaliero di San Giovanni di Gerusalemme di Rodi e di Maltacode: it is deprecated (Italia) Supremus Militaris Ordo Hospitalarius Sancti Ioannis Hierosolymitani Rhodiensis et Melitensiscode: la is deprecated (…

North-south state highway in Massachusetts, US Route 85Route 85 highlighted in redRoute informationMaintained by MassDOTLength21.01 mi[1] (33.81 km)Major junctionsSouth end Route 16 in MilfordMajor intersections I-495 in Milford Route 135 in Hopkinton Route 9 / Route 30 in Southborough US 20 in Marlborough I-290 / I-495 in Hudson North end Route 117 in Bolton LocationCountryUnited StatesStateMassachusetts Highway system Mas…

Professor of cognitive neuroimaging Gina RipponRippon in 2016BornGeorgina Mary Jane Rippon1950 (age 73–74)NationalityBritishScientific careerFieldsCognitive neuroimagingInstitutionsAston University, BirminghamThesisThe orienting reflex in normal and in schizophrenic subjects (1982) WebsiteOfficial website Gina Rippon (born 1950)[1] is a British neurobiologist and feminist. She is a professor emeritus of cognitive neuroimaging at the Aston Brain Centre, Aston University, B…

Finnish ice hockey player Ice hockey player Antti Roppo Born (1989-02-09)February 9, 1989Tampere, FinlandDied February 8, 2018(2018-02-08) (aged 28)Tampere, FinlandHeight 5 ft 11 in (180 cm)Weight 179 lb (81 kg; 12 st 11 lb)Position ForwardShot RightPlayed for IlvesHPKPlaying career 2004–2013 Antti Roppo (February 9, 1989 – February 8, 2018[citation needed]) was a Finnish professional ice hockey forward who played for Ilves and HPK of the S…
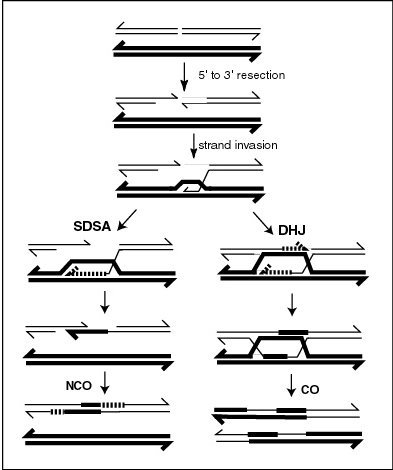
This artcicle is about the DNA helicase proteinSee also: Samsung Galaxy S Sgs1, also known as slow growth suppressor 1,[1] is a DNA helicase protein found in Saccharomyces cerevisiae. It is a homolog of the bacterial RecQ helicase. Like the other members of the RecQ helicase family, Sgs1 is important for DNA repair. In particular, Sgs1 collaborates with other proteins to repair double-strand breaks during homologous recombination in eukaryotes.[2] Meiosis A current model of meiot…

This article is part of a series aboutThomas Jefferson Early life and political career Early life and career Declaration of Independence Committee of Five Notes on the State of Virginia Minister to France Secretary of State First Party System 1796 election Vice presidency Personal life 3rd President of the United States Presidency First term 1800 election 1st inauguration Jeffersonian democracy Fiscal policy Twelfth Amendment Military Peace Establishment Act Yazoo land scandal Louisiana Purchase…

العلاقات البلغارية الهندوراسية بلغاريا هندوراس بلغاريا هندوراس تعديل مصدري - تعديل العلاقات البلغارية الهندوراسية هي العلاقات الثنائية التي تجمع بين بلغاريا وهندوراس.[1][2][3][4][5] مقارنة بين البلدين هذه مقارنة عامة ومرجعية للدولتين: وجه …
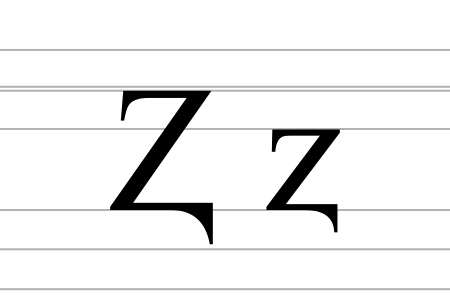
Letter of the Latin alphabet Ⱬ (minuscule: ⱬ, Unicode codepoints U+2C6B and U+2C6C, respectively) is a Latin letter Z with a descender. Z with descender in Doulos SIL It is used in pre-1983 romanization of the Uyghur language, transliterating ژ [ʒ], a pre-consonantal allophone of ج /dʒ/ (see Qona Yëziq), but occurring independently in a few words of Russian, Persian or Western origin (such as ⱬurnal from journal). It corresponds to the digraph zh in the current ULY standard. A…

2007 2017 Élections territoriales de 2012 à Saint-Martin 23 sièges du conseil territorial 18 et 25 mars 2012 Corps électoral et résultats Inscrits 17 882 Votants au 1er tour 9 304 52,03 % 5,7 Blancs et nuls au 1er tour 288 Votants au 2d tour 9 871 55,12 % 4,4 Blancs et nuls au 2d tour 283 Rassemblement Responsabilité Réussite – Alain Richardson Voix au 1er tour 3 077 34,13 % 2,2 Voix au 2e tour 5 45…

John Lawrence Ashbery Premio Pulitzer nel 1976 John Lawrence Ashbery (Rochester, 28 luglio 1927 – Hudson, 3 settembre 2017) è stato un poeta statunitense. Indice 1 Biografia 2 Raccolte poetiche 3 Note 4 Altri progetti 5 Collegamenti esterni Biografia Considerato il massimo esponente della scuola poetica newyorkese, svolge la propria opera artistica principalmente in un ambito meditativo, nel quale riesce a far confluire linguaggi e stili contemporanei, sovente derivati dal mondo dei mass-medi…

Alimin Sekretaris Jenderal Komite Sentral Partai Komunis IndonesiaMasa jabatanJuli 1950 – Januari 1951PresidenSukarnoPendahuluSardjonoPenggantiD.N. Aidit Informasi pribadiLahir1889 (1889)Surakarta, Hindia BelandaMeninggal26 Juni 1964(1964-06-26) (umur 74–75)Jakarta, IndonesiaPartai politikInsulindePartai Komunis Indonesia (PKI)Suami/istriHajjah MariahAnak2ProfesiEditor jurnal ModjopahitSunting kotak info • L • B Alimin bin Prawirodirdjo (1889 – 26 Juni 1964…

This article contains wording that promotes the subject in a subjective manner without imparting real information. Please remove or replace such wording and instead of making proclamations about a subject's importance, use facts and attribution to demonstrate that importance. (September 2017) (Learn how and when to remove this template message) Art museum, Sculpture park in Florida, United StatesThe Patricia & Phillip Frost Art MuseumFrost Art MuseumInteractive fullscreen mapEstablished1977L…

CST-100 StarlinerVéhicule spatial avec équipage Une vue d'artiste de l'amarrage du véhicule spatial CST-100 Starliner à la Station spatiale internationale.Fiche d'identité Organisation NASA Constructeur Boeing Type de vaisseau Capsule habitée Lanceur Atlas V N22 Base de lancement Cap Canaveral, LC-41 Premier vol 20 décembre 2019(sans équipage) Dernier vol 19-25 mai 2022(sans équipage) Nombre de vols 2 Statut En développement Caractéristiques Hauteur 5,0 m Masse à sec 6 350&…

Australian Open 1977 (dicembre)Singolare maschile Sport Tennis Vincitore Vitas Gerulaitis Finalista John Lloyd Punteggio 6-3, 7-6, 5-7, 3-6, 6-2 Tornei Singolare uomini donne Doppio uomini donne 1977 (gennaio) 1978 Voce principale: Australian Open 1977 (dicembre). Vitas Gerulaitis ha battuto in finale John Lloyd 6-3 7-6 5-7 3-6 6-2. Indice 1 Teste di serie 2 Tabellone 2.1 Legenda 2.2 Fase finale 2.3 Parte alta 2.3.1 Sezione 1 2.3.2 Sezione 2 2.4 Parte bassa 2.4.1 Sezione 3 2.4.2 Sezione 4…

Saudi Arabian novelist (born 1979) Mohammed Hasan AlwanBorn(1979-08-27)27 August 1979Riyadh, Saudi ArabiaNationalitySaudi ArabianGenreNovels, short stories Mohammed Hasan Alwan (born 27 August 1979) is a Saudi Arabian novelist.[1] He was born in Riyadh and studied Computer Information Systems at King Saud University, obtaining a bachelor's degree in 2002. He also obtained an MBA from the University of Portland, Oregon in 2008 and Ph.D from Carleton University, Ottawa in 2016.[2] …

Lead carbonate Names IUPAC name Lead(II) carbonate Other names Cerussite Identifiers CAS Number 598-63-0 Y ChemSpider 11234 ECHA InfoCard 100.009.041 EC Number 209-943-4 PubChem CID 11727 RTECS number OF9275000 UNII 43M0P24L2B Y CompTox Dashboard (EPA) DTXSID90883460 Properties Chemical formula PbCO3 Molar mass 267.21 g/mol Appearance White powder Density 6.582 g/cm3 Melting point 315 °C (599 °F; 588 K) (decomposes) Solubility in water 0.00011 g/(100…

Skadron Teknik 022Lanud Abdulrachman SalehNegara IndonesiaCabang TNI Angkatan UdaraTipe unitKomando TeknikBagian dariLanud Abdulrachman SalehSitus webwww.abdsaleh.mil.id Skadron Teknik 022 disingkat (Skatek 022) adalah unsur pelaksana pemeliharaan pesawat terbang yang berkududukan langsung di bawah Danlanud Abdulrachman Saleh, Malang Skatek bertugas menyelenggarakan pembinaan perneliharaan Alat Utama Sistem Senjata (Alutsista) beserta komponennya.[1] Dalam rangka pelaksanaan tugas t…

For-profit university in Waterbury, Connecticut, US This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages) This article may rely excessively on sources too closely associated with the subject, potentially preventing the article from being verifiable and neutral. Please help improve it by replacing them with more appropriate citations to reliable, independent, third-party sources. (February 2019) (L…


