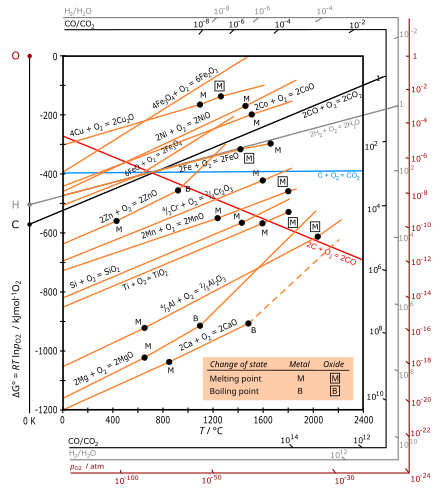
Ellingham diagram
|
Read other articles:

Deforestasi[pranala nonaktif permanen] di hutan Amazon terjadi di sepanjang akses jalan yang dibangun di kawasan hutanPengurusan Hutan di Indonesia adalah keseluruhan tindakan manajemen terhadap sumber daya hutan yang ada di Indonesia yang dilakukan dalam rangka mendapatkan totalitas barang-barang, manfaat-manfaat, dan nilai-nilai yang dapat diperoleh dengan tetap mempertahankan kelestariannya untuk generasi sekarang dan generasi yang akan datang. Ruang Lingkup Pengurusan Hutan Hutan di …

Bening dayak, gendongan bayi dengan hiasan manik-manik di desa Setulang Kalimantan Utara Bening dayak adalah salah satu alat tradisional untuk menggendong bayi bagi suku Dayak di Kalimantan utara yang turun temurun terutama di kalangan ibu-ibu Dayak Kenyah dan Dayak Bahau. Bening digunakan sebagai gendongan bayi suku Dayak pada saat anak umur 6 bulan hingga 1,5 tahun.[1] Cara menggunakan bening seperti menggunakan tas ransel, bening berada di punggung sang ibu dengan dua tali pengait ke …

International goals by Cristiano Ronaldo Ronaldo celebrates after scoring a penalty against New Zealand at the 2017 FIFA Confederations Cup in Saint Petersburg, Russia. Cristiano Ronaldo is a Portuguese professional footballer who has represented the Portugal national team since his debut on 20 August 2003 against Kazakhstan in a friendly.[1] He would later score his first international goal on 12 June 2004, during a UEFA Euro 2004 group stage match against Greece.[2] Since then,…

Dewan Perwakilan Rakyat DaerahProvinsi Kalimantan BaratPeriode 2019-2024JenisJenisUnikameral Jangka waktu5 tahunSejarahSesi baru dimulai30 September 2019PimpinanKetuaM. Kebing L. (PDI-P) sejak 13 November 2019 Wakil Ketua IPrabasa Ananatur (Golkar) sejak 13 November 2019 Wakil Ketua IISyarif Amin Muhammad (NasDem) sejak 13 November 2019 Wakil Ketua IIISuriansyah (Gerindra) sejak 13 November 2019 KomposisiAnggota65Partai & kursiPemerintah (55) PKB (5) G…
يفتقر محتوى هذه المقالة إلى الاستشهاد بمصادر. فضلاً، ساهم في تطوير هذه المقالة من خلال إضافة مصادر موثوق بها. أي معلومات غير موثقة يمكن التشكيك بها وإزالتها. (نوفمبر 2019) كأس آيسلندا 2009 تفاصيل الموسم كأس آيسلندا النسخة 50 البلد آيسلندا المنظم اتحاد آيسلندا لكرة القدم&…

Mode of delivering education to students who are not physically present Not to be confused with homeschooling or out-of-school learning. Distance education, also known as distance learning, is the education of students who may not always be physically present at school,[1][2] or where the learner and the teacher are separated in both time and distance.[3] Traditionally, this usually involved correspondence courses wherein the student corresponded with the school via mail.…

Fram Maret 1894 Fram adalah sebuah kapal penjelajah yang mengelilingi Kutub Utara dan Kutub Selatan.[1] Pembangunan Fram dirancang oleh Fridtjof Nansen, seorang penjelajah kutub dan ilmuwan Norwegia.[1] Pada saat itu ia merupakan kapal pertama yang dirancang untuk bertahan di musim salju saat di kutub.[1] Fram dirancang oleh Colin Archer, dengan menggunakan uang sebanyak 500.000 Krona Norwegia.[2] Bentuk rupa Fram berbobot 402 ton dan bertiang tiga.[1] Ia …

لمعانٍ أخرى، طالع باريس (توضيح). باريسالشعارالعلمشعار النبالةالتسميةسبب التسمية بَرِيش الاسم الرسمي Paris (بالفرنسية)[1] الشعار النصي Fluctuat nec mergitur (باللاتينية) الجوائز وسام جوقة الشرف من رتبة فارس[2] (9 أكتوبر 1900) رفيق التحرير صليب الحرب 1914-1918[3] موقع ال…

This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Miss Chinese International Pageant 1998 – news · newspapers · books · scholar · JSTOR (June 2009) (Learn how and when to remove this template message) Beauty pageant Miss Chinese International Pageant 1998DateJanuary 25, 1998PresentersEric Tsang, Lydia Shum, Emil ChauEntertainmentE…

Bagian dari seri tentangGereja Ortodoks TimurMosaik Kristos Pantokrator, Hagia Sofia Ikhtisar Struktur Teologi (Sejarah teologi) Liturgi Sejarah Gereja Misteri Suci Pandangan tentang keselamatan Pandangan tentang Maria Pandangan tentang ikon Latar belakang Penyaliban / Kebangkitan / KenaikanYesus Agama Kristen Gereja Kristen Suksesi apostolik Empat Ciri Gereja Ortodoksi Organisasi Otokefali Kebatrikan Batrik Ekumenis Tatanan keuskupan Klerus Uskup Imam Diakon Monastisisme Tingkatan mon…

Blank SpaceSingel oleh Taylor Swiftdari album 1989Dirilis10 November 2014 (2014-11-10)Format CD single digital download GenreElectropopDurasi3:51Label Big Machine Republic Pencipta Taylor Swift Max Martin Shellback Produser Max Martin Shellback Blank Space adalah sebuah lagu karya penyanyi-penulis lagu berkebangsaan Amerika Serikat Taylor Swift dari album studio kelima Swift, 1989. Lagu ini ditulis oleh Swift, Max Martin, dan Shellback. Lagu ini dirilis sebagai singel pada tanggal 10 Novemb…
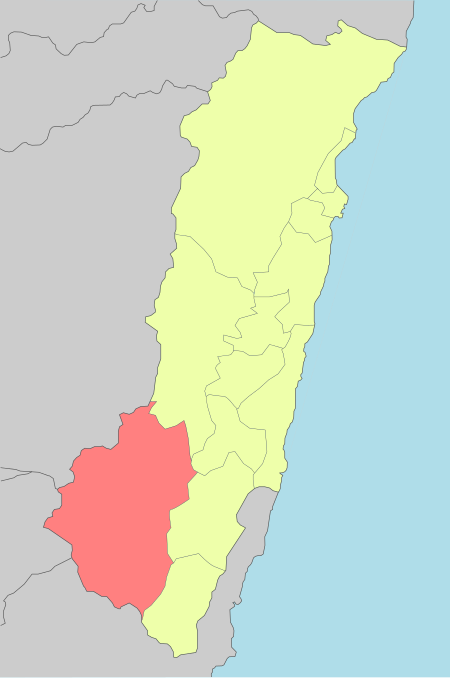
Place in Eastern Taiwan, TaiwanZhuoxi Township 卓溪鄉JhuosiCoordinates: 23°20′00″N 121°17′00″E / 23.33333°N 121.28333°E / 23.33333; 121.28333CountryTaiwanRegionEastern TaiwanGovernment • TypeTownshipArea • Total1,021.3130 km2 (394.3312 sq mi)Population (February 2023) • Total6,043Time zoneUTC+8 (CST)Post code982Subdivision6 VillagesWebsitewww.zhuo-xi.gov.tw Zhuoxi Township (Chinese: 卓溪鄉; piny…

追晉陸軍二級上將趙家驤將軍个人资料出生1910年 大清河南省衛輝府汲縣逝世1958年8月23日(1958歲—08—23)(47—48歲) † 中華民國福建省金門縣国籍 中華民國政党 中國國民黨获奖 青天白日勳章(追贈)军事背景效忠 中華民國服役 國民革命軍 中華民國陸軍服役时间1924年-1958年军衔 二級上將 (追晉)部队四十七師指挥東北剿匪總司令部參謀長陸軍總�…

Province in Cordillera, Philippines This article is about the province. For the ethnic group, see Ifugao people. For the language, see Ifugao language. For other uses, see Ifugao (disambiguation). Province in Cordillera Administrative Region, PhilippinesIfugaoProvinceProvince of Ifugao Clockwise from the top: Batad Rice Terraces, Bangaan Rice Terraces, Tappiya Falls, Banaue Rice Terraces, Ifugao stilt houses FlagSealLocation in the PhilippinesOpenStreetMapCoordinates: 16°50′N 121°10′Eþ…

Ribellione di Stonoparte Rivolta degli schiavi in nord AmericaAttuale sito della battagliaData9 settembre 1739 EsitoRivolta stroncata dagli schiavisti SchieramentiSchiavi africaniMilizia della Carolina del Sud ComandantiJemmy CatoWilliam Bull Effettivi80 circaMeno di 100 PerditeDa 35 a 50 morti in battaglia e altri giustiziatiCirca 20 uccisi in battaglia e altrettanti dagli schiavi durante la rivolta Voci di rivolte presenti su Wikipedia Manuale La ribellione di Stono (conosciuta anche come Cosp…

Indice 1 Teoria 2 Storia 3 Panoramica 4 Prostituzione, pornografia e BDSM 5 Critiche 6 Note 7 Bibliografia 8 Voci correlate 9 Collegamenti esterni La teoria queer è una teoria sociologica, incentrata sulla critica e il riesame dei concetti di sesso e genere, emersa all'inizio degli anni novanta negli Stati Uniti e in Europa. Teoria La teoria nacque in seno agli studi gay e lesbici, agli studi di genere e alla teoria femminista. Sulla scia delle tesi di Michel Foucault, Jacques Derrida e Julia K…

Kejuaraan Dunia BWF 2022Informasi turnamenEdisike-27LevelInternasionalJadwalpenyelenggaraan22–28 AgustusTempatpenyelenggaraanGimnasium Metropolitan TokyoTokyo, Jepang ← Huelva 2021 Kopenhagen 2023 → Pertandingan di Kejuaraan Dunia BWF 2022TunggalputraputriGandaputraputricampuranlbs Kejuaraan Dunia BWF 2022 (bahasa Inggris: 2022 BWF World Championships atau secara resmi dikenal dengan nama TotalEnergies BWF World Championships 2022 untuk alasan sponsor) adalah turnamen olahraga bulu tan…

Ираклеониты — ученики гностика Ираклеона (II век). Упоминаются как особая секта Епифанием и Августином; при крещении и миропомазании они соблюдали обряд помазания елеем и при этом произносили воззвания на арамейском языке, которые должны были освободить душу от власти �…

Football tournament season 1932 German championshipDeutsche FußballmeisterschaftReplica of the Viktoria trophyTournament detailsCountryGermanyDates8 May – 12 JuneTeams16Final positionsChampionsBayern Munich1st German titleRunner-upEintracht FrankfurtTournament statisticsMatches played15Goals scored72 (4.8 per match)Attendance292,000 (19,467 per match)Top goal scorer(s)Karl Ehmer (7 goals)← 19311933 → The 1932 German football championship, the 25th edi…

Juan Carlos Mareco and co-star Mariquita Gallegos in Otras pinochadas (1965). In this Spanish name, the first or paternal surname is Mareco and the second or maternal family name is Iturburúa. Juan Carlos Mareco Iturburúa (January 20, 1926 – October 8, 2009[1]) was a Uruguayan actor and radio and television talk show host. He achieved fame in Spain, Chile and Argentina from the 1960s onwards in comedy roles and as a television host in a variety of genres. Biograp…